The numeric value of the Avogadro constant expressed in reciprocal mole, a dimensionless number, is called the Avogadro number, sometimes denoted N or N0, which is thus the number of particles that are contained in one mole, exactly 6.022 140 76 × 1023. In tribute to him, the number of elementary entities (atoms, molecules, ions or other particles) in 1 mole of a substance, 6.022 140 76 × 10 23, is known as the Avogadro constant, one of the seven SI base units and represented by N A.
Avogadro's law (sometimes referred to as Avogadro's hypothesis or Avogadro's principle) or Avogadro-Ampère's hypothesis is an experimental gas law relating the volume of a gas to the amount of substance of gas present.[1] The law is a specific case of the ideal gas law. A modern statement is:
Avogadro's law states that 'equal volumes of all gases, at the same temperature and pressure, have the same number of molecules.'[1]
For a given mass of an ideal gas, the volume and amount (moles) of the gas are directly proportional if the temperature and pressure are constant.
The law is named after Amedeo Avogadro who, in 1812,[2][3] hypothesized that two given samples of an ideal gas, of the same volume and at the same temperature and pressure, contain the same number of molecules. As an example, equal volumes of gaseous hydrogen and nitrogen contain the same number of atoms when they are at the same temperature and pressure, and observe ideal gas behavior. In practice, real gases show small deviations from the ideal behavior and the law holds only approximately, but is still a useful approximation for scientists.
Mathematical definition[edit]
The law can be written as:
or
where
- V is the volume of the gas;
- n is the amount of substance of the gas (measured in moles);
- k is a constant for a given temperature and pressure.
This law describes how, under the same condition of temperature and pressure, equal volumes of all gases contain the same number of molecules. For comparing the same substance under two different sets of conditions, the law can be usefully expressed as follows:
The equation shows that, as the number of moles of gas increases, the volume of the gas also increases in proportion. Similarly, if the number of moles of gas is decreased, then the volume also decreases. Thus, the number of molecules or atoms in a specific volume of ideal gas is independent of their size or the molar mass of the gas.
Derivation from the ideal gas law[edit]
The derivation of Avogadro's law follows directly from the ideal gas law, i.e.
- ,
where R is the gas constant, T is the Kelvin temperature, and P is the pressure (in pascals).
Solving for V/n, we thus obtain
- .
Compare that to
which is a constant for a fixed pressure and a fixed temperature.
An equivalent formulation of the ideal gas law can be written using Boltzmann constantkB, as
- ,
where N is the number of particles in the gas, and the ratio of R over kB is equal to the Avogadro constant.
In this form, for V/N is a constant, we have
- .
If T and P are taken at standard conditions for temperature and pressure (STP), then k′ = 1/n0, where n0 is the Loschmidt constant.
Historical account and influence[edit]
Avogadro's hypothesis (as it was known originally) was formulated in the same spirit of earlier empirical gas laws like Boyle's law (1662), Charles's law (1787) and Gay-Lussac's law (1808). The hypothesis was first published by Amadeo Avogadro in 1811,[4] and it reconciled Dalton atomic theory with the 'incompatible' idea of Joseph Louis Gay-Lussac that some gases were composite of different fundamental substances (molecules) in integer proportions.[5] In 1814, independently from Avogadro, André-Marie Ampère published the same law with similar conclusions.[6] As Ampère was more well known in France, the hypothesis was usually referred there as Ampère's hypothesis,[note 1] and later also as Avogadro–Ampère hypothesis[note 2] or even Ampère–Avogadro hypothesis.[7]
Experimental studies carried out by Charles Frédéric Gerhardt and Auguste Laurent on organic chemistry demonstrated that Avogadro's law explained why the same quantities of molecules in a gas have the same volume. Nevertheless, related experiments with some inorganic substances showed seeming exceptions to the law. This apparent contradiction was finally resolved by Stanislao Cannizzaro, as announced at Karlsruhe Congress in 1860, four years after Avogadro's death. He explained that these exceptions were due to molecular dissociations at certain temperatures, and that Avogadro's law determined not only molecular masses, but atomic masses as well.
Ideal gas law[edit]
Boyle, Charles and Gay-Lussac laws, together with Avogadro's law, were combined by Émile Clapeyron in 1834,[8] giving rise to the ideal gas law. At the end of the 19th century, later developments from scientists like August Krönig, Rudolf Clausius, James Clerk Maxwell and Ludwig Boltzmann, gave rise to the kinetic theory of gases, a microscopic theory from which the ideal gas law can be derived as an statistical result from the movement of atoms/molecules in a gas.
Avogadro constant[edit]
Avogadro's law provides a way to calculate the quantity of gas in a receptacle. Thanks to this discovery, Johann Josef Loschmidt, in 1865, was able for the first time to estimate the size of a molecule.[9] His calculation gave rise to the concept of the Loschmidt constant, a ratio between macroscopic and atomic quantities. In 1910, Millikan'soil drop experiment determined the charge of the electron; using it with the Faraday constant (derived by Michael Faraday in 1834), one is able to determine the number of particles in a mole of substance. At the same time, precision experiments by Jean Baptiste Perrin led to the definition of Avogadro's number as the number of molecules in one gram-molecule of oxygen. Perrin named the number to honor Avogadro for his discovery of the namesake law. Later standardization of the International System of Units led to the modern definition of the Avogadro constant.
Molar volume[edit]

Taking STP to be 101.325 kPa and 273.15 K, we can find the volume of one mole of gas:
For 101.325 kPa and 273.15 K, the molar volume of an ideal gas is 22.4127 dm3⋅mol−1.
See also[edit]
- Boyle's law – Relationship between pressure and volume in a gas at constant temperature
- Charles's law – Relationship between volume and temperature of a gas at constant pressure
- Gay-Lussac's law – Relationship between pressure and temperature of a gas at constant volume.
- Ideal gas – Mathematical model which approximates the behavior of real gases
Notes[edit]
- ^First used by Jean-Baptiste Dumas in 1826.
- ^First used by Stanislao Cannizzaro in 1858.
References[edit]
- ^ abEditors of the Encyclopædia Britannica. 'Avogadro's law'. Encyclopædia Britannica. Retrieved 3 February 2016.CS1 maint: extra text: authors list (link)
- ^Avogadro, Amedeo (1810). 'Essai d'une manière de déterminer les masses relatives des molécules élémentaires des corps, et les proportions selon lesquelles elles entrent dans ces combinaisons'. Journal de Physique. 73: 58–76.English translation
- ^'Avogadro's law'. Merriam-Webster Medical Dictionary. Retrieved 3 February 2016.
- ^Avogadro, Amadeo (July 1811). 'Essai d'une maniere de determiner les masses relatives des molecules elementaires des corps, et les proportions selon lesquelles elles entrent dans ces combinaisons'. Journal de Physique, de Chimie, et d'Histoire Naturelle (in French). 73: 58–76.
- ^Rovnyak, David. 'Avogadro's Hypothesis'. Science World Wolfram. Retrieved 3 February 2016.
- ^Ampère, André-Marie (1814). 'Lettre de M. Ampère à M. le comte Berthollet sur la détermination des proportions dans lesquelles les corps se combinent d'après le nombre et la disposition respective des molécules dont les parties intégrantes sont composées'. Annales de Chimie (in French). 90 (1): 43–86.
- ^Scheidecker-Chevallier, Myriam (1997). 'L'hypothèse d'Avogadro (1811) et d'Ampère (1814): la distinction atome/molécule et la théorie de la combinaison chimique'. Revue d'Histoire des Sciences (in French). 50 (1/2): 159–194. doi:10.3406/rhs.1997.1277. JSTOR23633274.
- ^Clapeyron, Émile (1834). 'Mémoire sur la puissance motrice de la chaleur'. Journal de l'École Polytechnique (in French). XIV: 153–190.
- ^Loschmidt, J. (1865). 'Zur Grösse der Luftmoleküle'. Sitzungsberichte der Kaiserlichen Akademie der Wissenschaften Wien. 52 (2): 395–413.English translation.
Due to the incredibly small size of the basic particles of matter there are fantastic numbers of them in the masses used in everyday life. Amedeo Avogadro is the scientist most credited with work done in this domain. | Amedeo Avogadro |
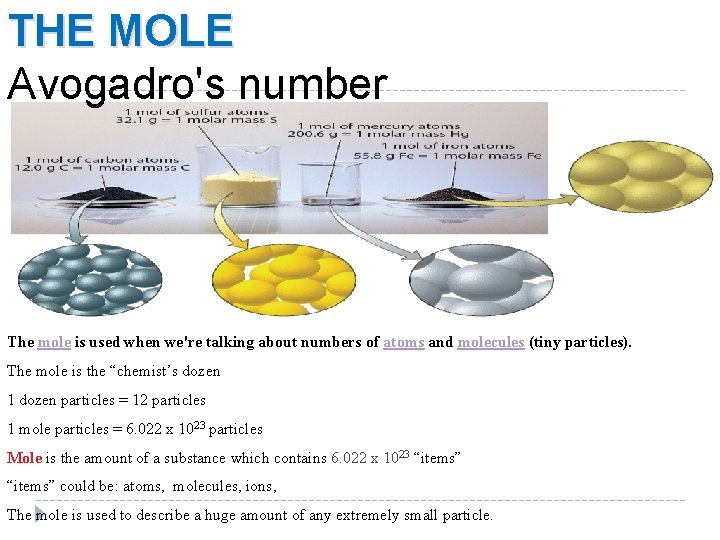
Avogadro's number
Avogadro defined the number of particles of a substance that have a mass equivalent to the relative mass of that substance. He found that the number of atoms in 12 g of carbon was equal to approximately 6.02 x 1023
This number was given his name - the Avogadro constant.
Avogadro's number = 6.02 x 1023

An Avogadro number of particles = 6.02 x 1023 particles
The Avogadro number or constant may be represented by the letter 'L' and when dealing with this number of particles we say that there is an 'amount' of 1 mole.
Avagro Number
1 mole of any substance contains an Avogadro number of particles of that substance = 6.02 x 1023 particles
How To Calculate Avogadro's Constant
Examples: 1 mole of iron contains 6.02 x 1023 iron atoms 1 mole of chlorine gas contains 6.02 x 1023 chlorine molecules 1 mole of electrons contains 6.02 x 1023 electrons (also called a Faraday of charge) |
Avogadro's Number Equation
Note that the Avogadro constant simply describes a specific number of any particle, be they atoms, molecules, electrons or even armchairs!